Stacking three layers of graphene with a twist speeds up electrochemical reactions
Tri-layer may be better than bi-layer for manufacturing, improving the speed and capacity of electrochemical and electrocatalytic devices

Tri-layer may be better than bi-layer for manufacturing, improving the speed and capacity of electrochemical and electrocatalytic devices
Expert

Three layers of graphene, in a twisted stack, benefit from a similar high conductivity to “magic angle” bilayer graphene but with easier manufacturing—and faster electron transfer. The finding could improve nano electrochemical devices or electrocatalysts to advance energy storage or conversion.
Graphene—a single layer of carbon atoms arranged in a hexagonal lattice—holds unique properties, including high surface area, excellent electrical conductivity, mechanical strength and flexibility, that make this 2D material a strong candidate for increasing the speed and capacity of energy storage.
Twisting two sheets of graphene at a 1.1° angle, dubbed the “magic angle”, creates a “flat band” structure, meaning the electrons across a range of momentum values all have roughly the same energy. Because of this, there is a huge peak in the density of states, or the available energy levels for electrons to occupy, at the energy level of the flat band which enhances electrical conductivity.
Recent work experimentally confirmed these flat bands can be harnessed to increase the charge transfer reactivity of twisted bilayer graphene when paired with an appropriate redox couple—a paired set of chemicals often used in energy storage to shuttle electrons between battery electrodes.
Adding an additional layer of graphene to make twisted trilayer graphene yielded a faster electron transfer compared to bilayer graphene, according to an electrochemical activity model in a recent study by University of Michigan researchers.
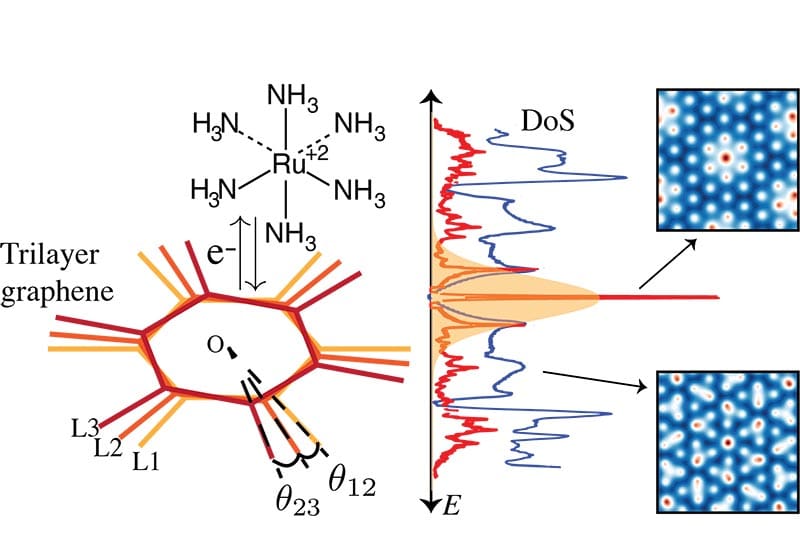
“We have discovered highly flexible and enhanced charge transfer reactivity in twisted trilayer graphene, which is not restricted to specific twist angles or redox couples,” said Venkat Viswanathan, an associate professor of aerospace engineering and corresponding author of the study published in the Journal of the American Chemical Society.
Stacking three layers of graphene introduced an additional twist angle, creating “incommensurate”, meaning non-repeating patterns, at small-angle twists—unlike bilayer graphene which forms repeating patterns. Essentially, when adding a third layer, the hexagonal lattices do not perfectly align.
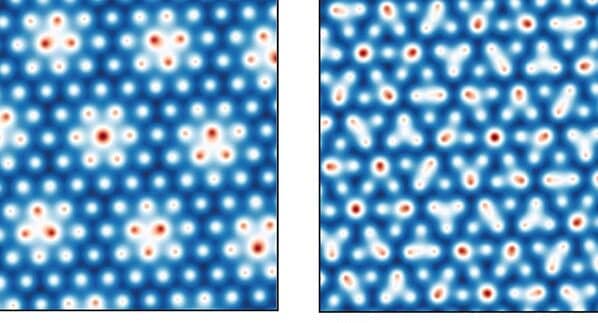
Left: Periodic atomic structure of the magic angle in trilayer graphene. Right: Non-periodic atomic structure of the incommensurate angle in trilayer graphene. Image credit: Babar et al. 2024
[Left: Hexagonal repeating pattern of blue, orange, and yellow. Right: A non-repeating hexagonal pattern.]
At room temperature, these non-repeating patterns have a wider range of angles with high density of states away from the flat bands, increasing electrical conductivity comparable to those predicted at the magic angle.
“This discovery makes fabrication easier, avoiding the challenge of ensuring the precise twist angle that bilayer graphene requires,” said Mohammad Babar, a doctoral student of mechanical and aerospace engineering and first author of the study.
As a next step, the researchers plan to verify these findings in experiments and potentially discover even higher activity in multi-layer twisted 2D materials for a wide range of electrochemical processes such as redox reactions and electrocatalysis.
“Our work opens a new field of kinetics in 2D materials, capturing the electrochemical signatures of commensurate and incommensurate structures. We can now identify the optimal balance of charge transfer reactivity in trilayer graphene for a given redox couple,” said Babar.
This work was funded in part by the Simons Foundation (Award no. 896626) and computational resources were provided by the Extreme Science and Engineering Discovery Environment (Award No. TG-CTS180061).
Additional co-authors: Ziyan Zhu of the SLAC National Accelerator Laboratory, Rachel Kurchin of Carnegie Mellon University, Efthimios Kaxiras of Harvard University.
Full citation: “Twisto-Electrochemical Activity Volcanoes in Trilayer Graphene,” Mohammad Babar, Ziyan Zhu, Rachel Kurchin, Efthimios Kaxiras, and Venkatasubramanian Viswanathan, Journal of American Chemical Society (2024). DOI: 10.1021/jacs.4c03464

Left: Trilayer graphene twisted by θ12 and θ23, exchanging an electron from a redox couple, ruthenium hexamine. Center: Density of states (DoS) of trilayer graphene at the magic angle in red (1.1°/4.7°) and the incommensurate angle in blue (2.7°/4.7°). Right: Periodic atomic structure of the magic angle and non-periodic atomic structure of the incommensurate angle. Image credit: Babar et al. 2024